Ministry of Science and Technology invites Concept Proposals for CAR T Cell therapy
Delhi: Through a recent notice, Ministry of Science and Technology has invited Concept Proposals on approach to developing CAR T Cell therapy at an affordable price in India. The last date for the submission of Concept Proposal is 15th March 2020.
The proposal should be submitted in the prescribed format (4 hard copies) to the following address i.e, Dr Niloo Srivastava, Scientist 'E', Department of Biotechnology, Block-2, Room No.621, 6th floor, CGO Complex, Lodhi Road, New Delhi – 110003 along with a soft copy (both word and pdf format) to be emailed to nsrivastava.dbt@nic.in and manoj.kr09@gov.in.
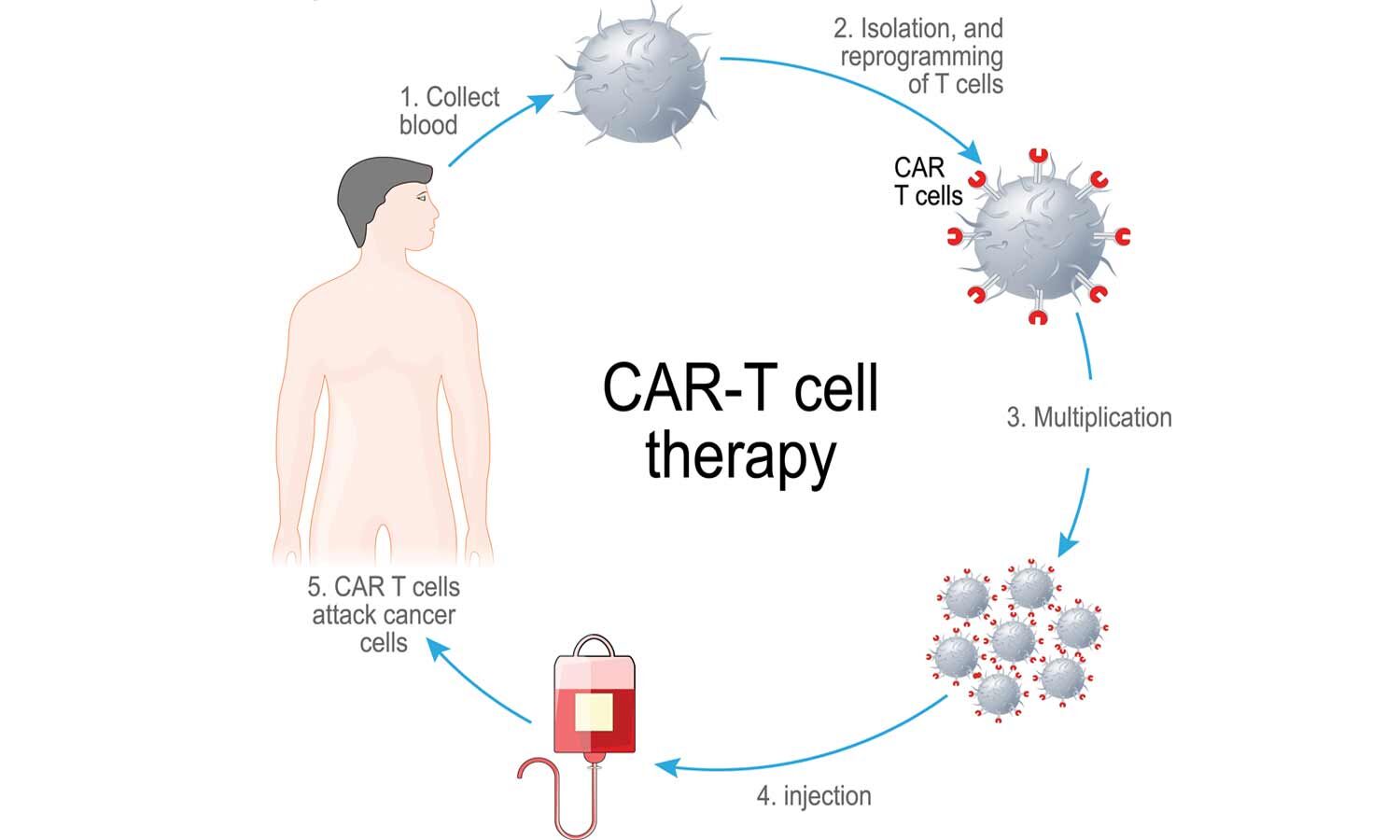
CAR-T cell-based therapy has emerged as a breakthrough in cancer treatment. It is a type of treatment in which a patient's T cells are changed in the laboratory enabling them to specifically attack cancer cells and these cells are then infused back into the patient's body. Clinical trials conducted globally have shown promising results in endstage patients especially in patients suffering from Acute Lymphocytic Leukemia.
Though this technology has a remarkable therapeutic potential for cancer patients, at present this technology is very expensive and not available in India. The challenge, therefore, is to develop this technology in a cost-effective manner and make it available for the patients.
The goal of this Call is to support milestone-driven multi-institutional/multi-investigator proposals focused on developing CAR-T technology in India. Accordingly Request for Concept Proposals are invited on "Approach to develop CAR T Cell therapy at an affordable price in India" for consideration under the Stem Cells & Regenerative Medicine Programme of the Department. Applications are expected to have defined Milestones and Timelines detailing how the project will move forward with identified deliverables and expected outcomes.
The focus of the Concept proposal may be on the following, but not limited to:
1. Basic, early and late translational research studies to develop CAR-T technology in India and also to improve the existing CAR-T cell therapy by exploring new Antigen targets and to expand the CAR T cell therapy for other haematological malignancies and solid tumours.
2. Development of clinical-grade plasmids/vectors for gene transfer
3. Pre-clinical studies and Clinical trials for CAR-T technology
4. Mitigating possible adverse events, both short and long term for eg., specific modifications in CAR constructs to reduce the post-transplantation incidences of life-threatening cytokine release syndrome (CRS) and neurotoxicity.
5. Methods to scale-up the production of CAR T cells in an efficient, and effective manner.
Collaborative proposals may be submitted by public and private universities, colleges, Institutes, non-profit organizations (recognized by DSIR as a Scientific and Industrial Research Organization (SIRO))
1. The Academic centres if not a medical institute must be linked with a medical centre/hospital preferably within the same city so that patient samples could be easily obtained for the generation of CAR T cells.
2. These centres/institutes/hospitals should have well-defined teams of basic scientists and clinicians. Basic science team should have a strong background in the areas of molecular biology, cell biology and immunology, whereas the clinical team should have expertise in the management of bone marrow transplant patients and a track record of performing clinical trials.
3. Existing GMP facility at one of these centres/institutes for manufacturing of CAR - T cells.
4. All Concept proposals must adhere to statutory regulatory requirements.
For any queries, contact Dr Manoj Kumar, Scientist-C, Email-manoj.kr09@gov.in / Dr Niloo Srivastava, Scientist-E, Email- nsrivastava.dbt@nic.in
The ministry has also issued Performa for the same
1.(i) Project Title:
(ii) Specific Area of your proposal:
(iii) Name of the Coordinating institution:
(iv) Details of Partnering institutions:
(iv) Names(s) of the Investigator(s),
(v) Institute address, Contact numbers, emails etc.
2. Indicate the category of domain expertise and potential of each PIs (Basic and/or clinical research). Multifaceted and multi-institutional collaborations encouraged.
3. Scientific Hypothesis and key questions to be addressed and Primary Objectives (100 words)
4. Detailed work plan i.e., how will you test the hypothesis/approach towards the development of technology and solutions? (500 Words)
5. What is the novelty in your approach?
6. Feasibility of doing the study in your present institution/workplace:
7. Tentative budget (under the headings Non-recurring/Manpower/Recurring).
8. List 4-6 statements on expected deliverables (a) study outcomes (or) translational outcomes.
9. Professional Experiences and Training relevant to the project
10. The CV of the investigators as per format (Annexure I).
11. Any other highlights
For the full details click on the following link:
PDF Link: https://business.medicaldialogues.in/pdf_upload/pdf_upload-125180.pdf
Medical Dialogues Bureau consists of a team of passionate medical/scientific writers, led by doctors and healthcare researchers. Our team efforts to bring you updated and timely news about the important happenings of the medical and healthcare sector. Our editorial team can be reached at editorial@medicaldialogues.in. Check out more about our bureau/team here

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd