Bevetex Vs Bevatas: Delhi HC junks Sun Pharma arm plea against Intas Pharma over trademark
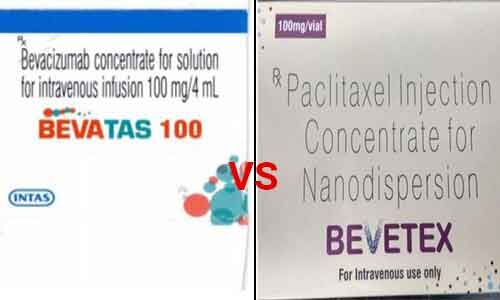
New Delhi: In a major setback to drug giant Sun Pharmaceuticals Ltd.'s arm, Sun Pharma Laboratories Ltd., the Delhi high court has recently dismissed a petition filed by the company related to the alleged violation of the trademark of its cancer drug
Sun pharma had knocked on the doors of the high court challenging the order by a trial court that dismissed its plea against Intas Pharmaceutical. The plea sought to direct to restrict the sale of allegedly infringed trademark 'BEVATAS' by Intas Pharma that is deceptively similar to that of Sun Pharma's trademark 'BEVETEX'.
In its plea, Sun Pharma stated that Bevetex was invented in the year 1983 and contains Molecule/Salt PACLITAXEL and is a scheduled drug used for the treatment of Breast Cancer, Non-Small Cell Lung cancer and Pancreatic cancer. Sun Pharma has been selling the drug since 2015.
However, in 2017, it came across a medicine under the mark BEVATAS sold by Intas Pharma. Upon enquiry, it was learnt that the drug was launched in October 2017.
The drugmaker submitted that being the proprietor of the trademark BEVETEX, it has the exclusive right to use the said trademark and ought to be protected against imitation, confusion, deception, dilution and unfair competition by competitors in trade.
Sun Pharma submitted that Intas medicine under the impugned mark although a cancer drug contains a different salt namely BEVACIZUMAB and is used for the treatment of Colorectal cancer, Ovarian cancer, Cervical cancer, Lung cancer and recurrent gliolastoma (a type of brain tumour). Nevertheless, the wrong administration of the drug can prove fatal, the drugmaker added.
"Intas Pharma's impugned mark BEVATAS is structurally, visually and phonetically similar to its trademark BEVETEX and competing medicines are sold in the same dosage form i.e. injections and are sold at similar prices and that such adoption also amounts to unfair trade practice, unfair competition and dilution and such act also amounts to misrepresentation and misappropriation of Sun Pharma's goodwill in the trademark BEVETEX," it further contended in its plea/
The petition sought to restrict the impugned mark BEVATAS claiming to protect the public interest at large and protect the consumers against confusion and deception.
Responding to the contentions, Intas submitted that Sun Pharma has already admitted that BEVETEX and BEVATAS contain totally different molecule formulations/salts and that BEVETEX is a product of the chemical formulation PACLITAXEL, which is a synthetic chemical compound and whereas, BEVATAS is a product of a biosimilar BEVACIZUMAB, which is rDNA in nature.
It further submitted that there was no similarity in both the marks "BEVETEX" and "BEVATAS" which are entirely different and there is no likelihood of confusion or deception and/or similarity between the two marks amongst the customers and that the two marks are totally different, dissimilar and distinct and that there cannot be any likelihood of confusion and that the said marks are visually and phonetically dissimilar.
Intas added that the company had also applied for the registration of its trademark and that Sun Pharma's Trade Mark when examined by the Examiner of Trademarks, he did not cite any conflicting trademark and the application was accepted for advertisement and even the trademarks office did not find any similarity between the trademarks Bevetex and Bevatas.
"The marks BEVETEX and BEVATAS are totally different, dissimilar and distinct and that the drug formulations of the marks are completely different along with mode of administration and that there cannot be likelihood of confusion as these drugs are Schedule H drugs and cannot be sold off the shelf and the indications and effect of both the drugs are different and is known to the experts and medical practitioners who prescribe these drugs," Intas added.
Intas further added that prior to moving the court, the entire submissions were closely observed by the trial court, that dismissed Sun Pharma's application. The trial court noted that the entire controversy was centred around whether Intas Pharma's trademark 'BEVATAS' is deceptively similar to that of Sun Pharma's trademark 'BEVETEX'.
As per the trial court's opinion, "It is not uncommon, rather, in pharmaceutical trade, it is a normal practice to have prefixes or suffixes linked to chemical salt or compound across various similar products manufactured by different companies. It is also common to have the abbreviated name of the company in conjunction with such prefix or suffix of the salt to indicate the source of the product. In view of the pharmaceutical trade, one finds the name of various drugs almost similar to each other, having common prefixes of suffixes, for the reason that the name of the drug conveys as to which salt/ compound it is a derivative of the drug."
Examining the entire matter and the trial court's order, Justice Anu Malhotra while referring to a number of similar cases taken by the Supreme court subsequently noted;
"The Court is not to re-assess the material and seek to reach to a conclusion different from the one reached by the Court below if one reached by that Court was reasonably possible on the material available and the Appellate Court would not normally be justified in interfering with the exercise of discretion under appeal solely on the ground that if it had considered the matter at the trial stage it would have come to contrary conclusion and that if the discretion that has been exercised by the learned trial Court has been reasonably exercised and in a judicial manner that the Appellate Court may or would have taken a different view, may not justify interference with the trial Courts exercise of discretion."
The court finally held;
"In the instant case, as it is apparent, it cannot be contended on behalf of Sun Pharma arm that there has been an unreasonable or un-judicious exercise of discretion by the learned trial Court vide the impugned order dated 17.09.2018 in the non-grant of the prayer for the ad-interim injunction as prayed by the drugmaker. In the circumstances, there is no merit in the appeal against the said impugned order, which is thus dismissed."
You can read the full order by clicking on the following link
Farhat Nasim joined Medical Dialogue an Editor for the Business Section in 2017. She Covers all the updates in the Pharmaceutical field, Policy, Insurance, Business Healthcare, Medical News, Health News, Pharma News, Healthcare and Investment. She is a graduate of St.Xavier’s College Ranchi. She can be contacted at editorial@medicaldialogues.in Contact no. 011-43720751 To know about our editorial team click here

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd